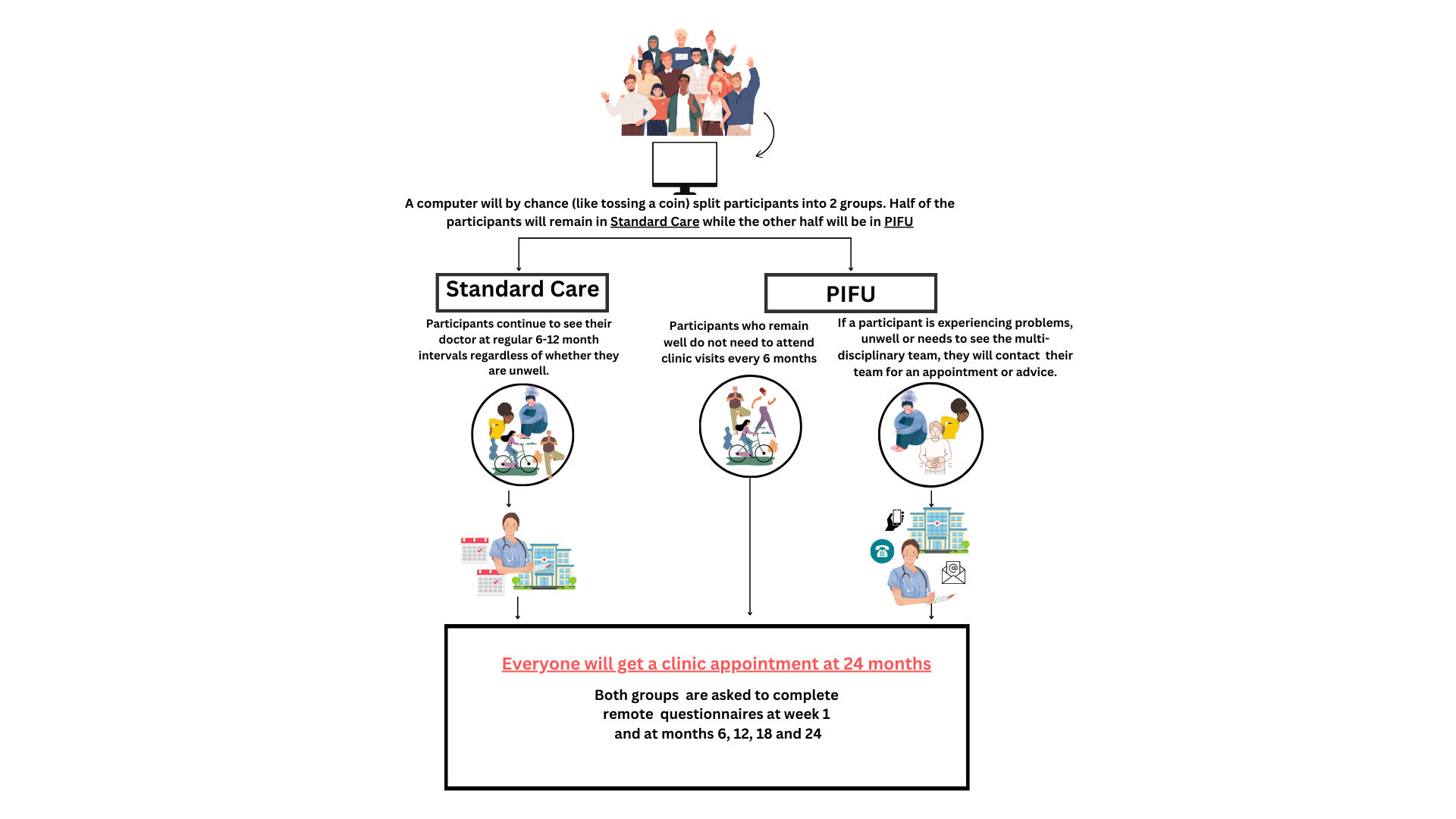
The TaILOR trial aims to study the clinical and cost-effectiveness of a patient-initiated follow-up (PIFU) strategy compared to traditional care pathways in people with inflammatory arthritis treated with long-term immune-suppressing therapies.
Inflammatory arthritis usually prevents people from doing things through causing joints to become swollen and painful, or their spine to become stiff. People with arthritis usually require long term treatment with drugs (medications) that affect their immune systems, because of this these individuals are typically reviewed in outpatient clinics every 6-12 months to check how participants are and that the drugs that should be helping their arthritis are not causing them different issues. However, many of these appointments are unnecessary as patients are well at the time of follow up.
NHS England has recently proposed that many people with inflammatory arthritis should no longer have routine follow up appointments, but be seen if and when they have a problem related to their arthritis such as a flare – they have called this ‘Patient-Initiated Follow Up’ or PIFU.
Our aim is to test whether PIFU is better than traditional routine follow-up appointments for patients over 24 months.
Overview of the TaILOR study in pictures