Participants will be randomised to patient initiated follow up (PIFU) or standard care with routine remote or face to face follow-up appointments booked at 6-12 months in accordance with local practice.
Eligibility and Inclusion Criteria
- Aged 18 years or over.
- Diagnosis of inflammatory arthritis (RA, PsA, axSpA, undifferentiated arthritis) for at least 2 years.
- Stable disease: defined as a level of disease control that the physician feels is suitable for PIFU; on the same conventional, targeted synthetic or biologic DMARD(s), or no treatment, for at least the previous 3 months; and with no escalation in therapy planned.
- Able to contact the Rheumatology team when required.
- Suitable for PIFU in the opinion of their consultant.
- Willing and able to give consent and comply with study procedures.
Eligibility to be invited to take part in the study will initially be determined by the patient’s usual care team as part of clinical care visit/assessment, by a suitably qualified and experienced individual who has been delegated to do so by the PI.
Once informed consent has been obtained and eligibility confirmed at the baseline visit, participants will complete questionnaires to collect patient-reported outcomes (PRO). Clinical data will also be collected from the medical notes. Participants will then be randomised to PIFU or standard care and will attend clinic appointments as per this allocation.
All participants will have a routine follow-up visit at 24 months during which routine clinical assessments will be carried out and data transcribed into the CRFs. During the 2 year intervention period, PRO data will be collected via remote completion of questionnaires.
Exclusion Criteria
A patient will not be eligible for the study if ANY of the following apply:
- Currently or previously on PIFU for inflammatory arthritis
- Safeguarding/consent/capacity concerns (using General Medical Council guidance).
- Health literacy concerns from the treating clinician related to inflammatory arthritis.
- Women who are pregnant or planning to start a family *1
- Currently undergoing radiotherapy, immunotherapy or chemotherapy for malignancy.
- Patients on end-of-life care pathways.
*1. Reported by patient. No pregnancy testing is required.
Rationale for Inclusion and Exclusion Criteria
Individuals currently or previously on a PIFU pathway for inflammatory arthritis have been excluded. The study aims to investigate the systematic rollout of PIFU (including education) so including participants with PIFU experience in inflammatory arthritis may unnecessarily influence outcomes.
Key inclusion/exclusion criteria are taken from the NHS England guidance for PIFU in inflammatory arthritis. Additional exclusion criteria (pregnancy, malignancy and end of life) were added as key safety issues raised by experienced clinicians in developing the protocol. Women who are pregnant or planning to start a family have been excluded due to clinical concerns about leaving patients with less support during pregnancy when their arthritis disease activity may fluctuate significantly and additional monitoring is required.
PIFU Infographic and example Timeline
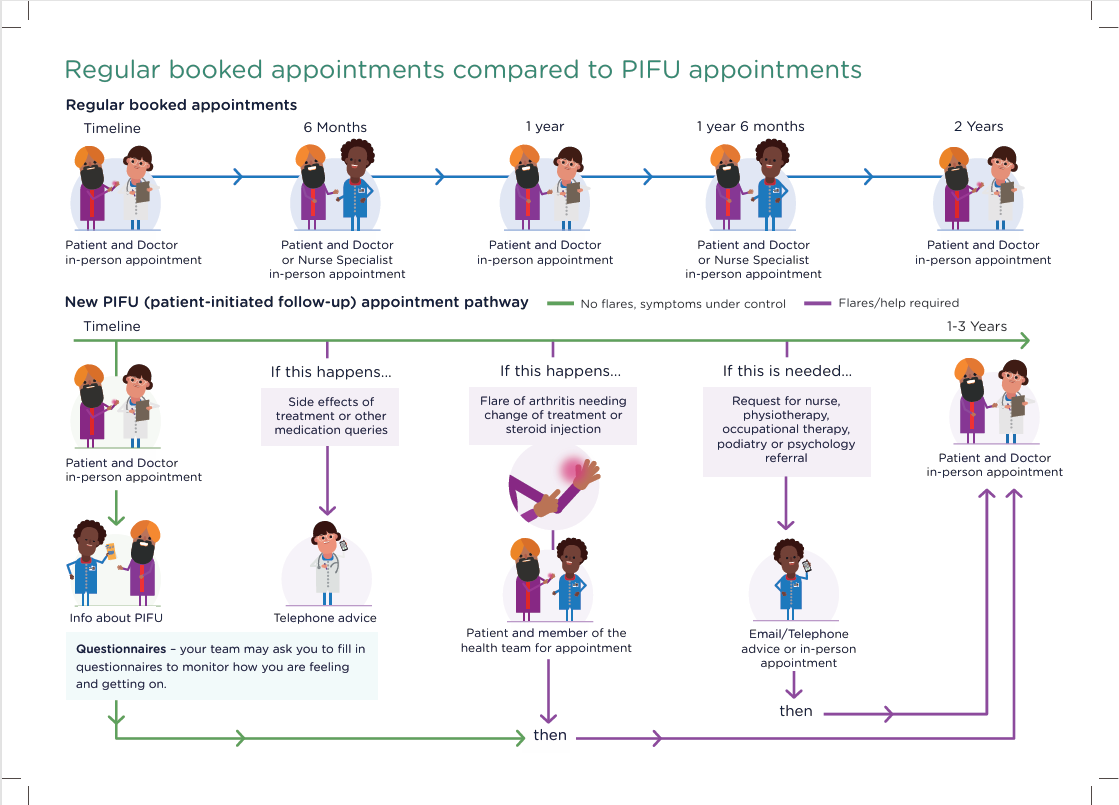
PIFU Infographic and example Timeline PIFU A4 Infographic Folded_Hi-res with print marks Final 160924.pdf (rheumatology.org.uk)
FAQs for Clinicians PIFU Clinicians Handbook Final 160924 (1).pdf (rheumatology.org.uk)
Rheumatology Advances in Practice Patient Initiated Follow-Up (PIFU): how can rheumatology departments start to reap the benefits? A consensus document | Rheumatology Advances in Practice | Oxford Academic (oup.com)
Please Note; All resources have been developed by BSR (British Society for Rheumatology)



