Overview
At the moment in the NHS, individuals who have inflammatory arthritis are regularly seen in hospital out-patient clinics to allow rheumatology teams to check in on how their arthritis is and any impact it is having on individuals.
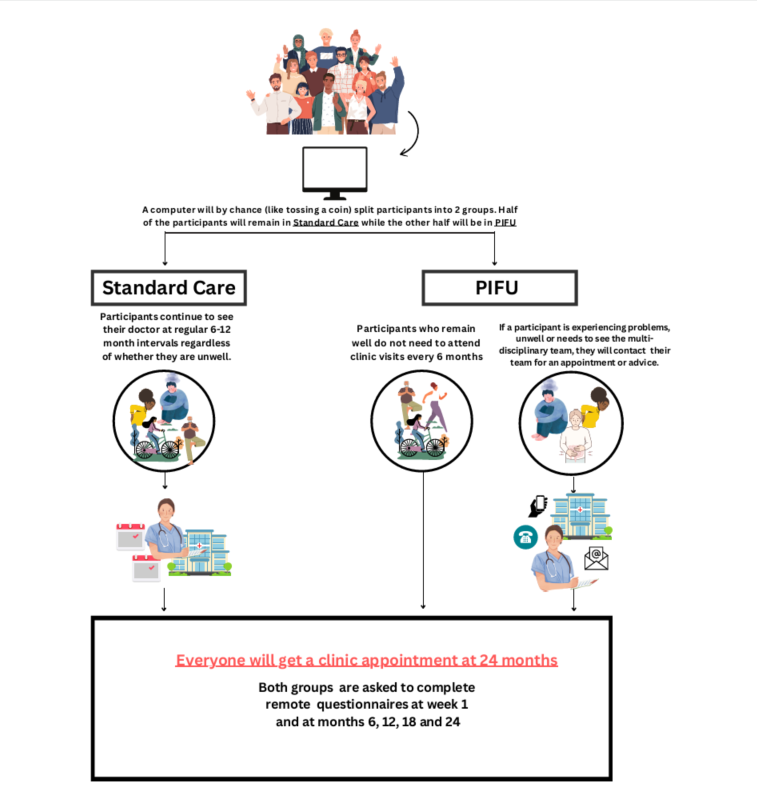
Some doctors, nurses and individuals with arthritis think it might be better if rather than go to hospital every 6-12 months for an out-patient appointment when you might have no issues, instead you are only seen in hospital every 24 months. In addition, patients are given information on how to contact their rheumatology team if they become unwell during this period. This is referred to as patient initiated follow up (PIFU).
The TaILOR trial aims to understand how PIFU compares to traditional care follow up in people with inflammatory arthritis. Half of those that agree to take part in the TaILOR study will have standard out-patient appointments every 6-12 months and the other half will have no planned out-patient appointments for the next 24 months.
In both groups, participants will be asked about their general health, arthritis symptoms at 4 time points over the 24 months to enable researchers to ultimately find out which is the best way for those with arthritis to be seen within the NHS.
To join the study you must be a patient at one of the hospitals taking part, a full list of hospitals can be viewed via our All Sites page
For further information you can contact the Rheumatology team at your preferred site, if you are interested in taking part.
The BSR (British Society for Rheumatology) have developed the following resources for patients about PIFU which you many be interested in looking at.
PIFU Infographic and example Timeline PIFU A4 Infographic Folded_Hi-res with print marks Final 160924.pdf (rheumatology.org.uk)
FAQs For Patients PIFU FAQ Document Final 160924 (1).pdf (rheumatology.org.uk)
Video Resources to Support Patients
Patient initiated follow-up (youtube.com)
Patient Initiated Follow up video resources translated
https://f.io/qDEWPV7m - Punjabi
https://f.io/dETpBNNn - Romanian
https://f.io/xsqGvDxr - Urdu
https://f.io/YrI35dBp - Polish
https://f.io/mnIq8PeM - Cantonese



